Cefadroxil is a first-generation cephalosporin antibiotic used to treat a variety of bacterial infections, including those affecting the skin, throat, and urinary tract. It works by inhibiting bacterial cell wall synthesis, leading to the destruction of the bacteria. Maintaining the pharmaceutical purity of Cefadroxil is essential to ensure its safety, effectiveness, and compliance with regulatory standards. Impurity profiling plays a key role in identifying and controlling unwanted by-products that may arise during manufacturing, storage, or degradation. Here are some of its known impurities listed below.
Showing 1 - 2 of 2 products
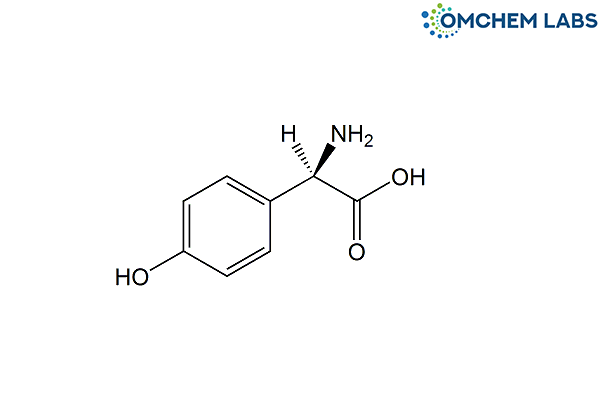
Catalogue No : CEFA-OCL-001
CAS No : 22818-40-2
In Stock
Synonyms
(2R)-2-Amino-2-(4-hydroxyphenyl)acetic acid
(2R)-2-Amino-2-(4-hydroxyphenyl)acetic acid
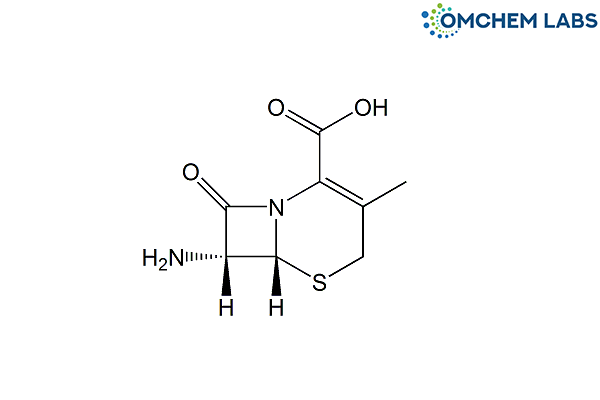
Catalogue No : CEFA-OCL-002
CAS No : 22252-43-3
In Stock
Synonyms
7-ADCA Cefadroxil USP RC B (6R,7R)-7-Amino-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
7-ADCA Cefadroxil USP RC B (6R,7R)-7-Amino-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
