Bupropion is an atypical antidepressant primarily prescribed for major depressive disorder (MDD) and smoking cessation, commonly marketed under brand names such as Wellbutrin and Zyban. Unlike selective serotonin reuptake inhibitors (SSRIs), Bupropion primarily acts on norepinephrine and dopamine neurotransmitters, offering a unique therapeutic profile with minimal sexual side effects and reduced risk of weight gain. Ensuring the pharmaceutical purity of Bupropion is essential for maintaining its safety, efficacy, and compliance with regulatory standards. Impurity profiling plays a key role in detecting and managing potential by-products that may arise during manufacturing, storage, or degradation. Here are some of its known impurities listed below.
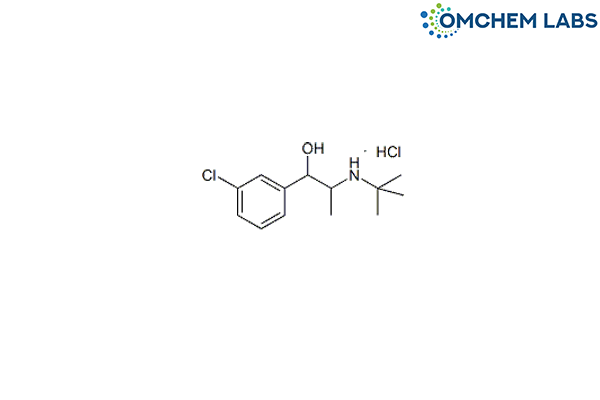
CAS No : 1396889-62-5
3-Chloro-α-[1-[(1,1-dimethylethyl)amino]ethyl]-benzenemethanol hydrochloride
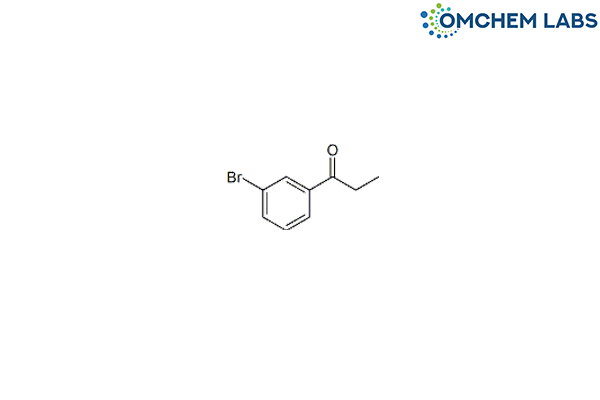
CAS No : 19829-31-3
m-Bromopropiophenone
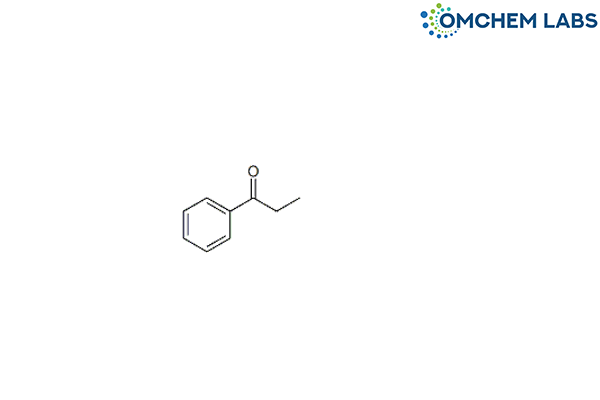
CAS No : 93-55-0
1-Phenyl-1-propanone
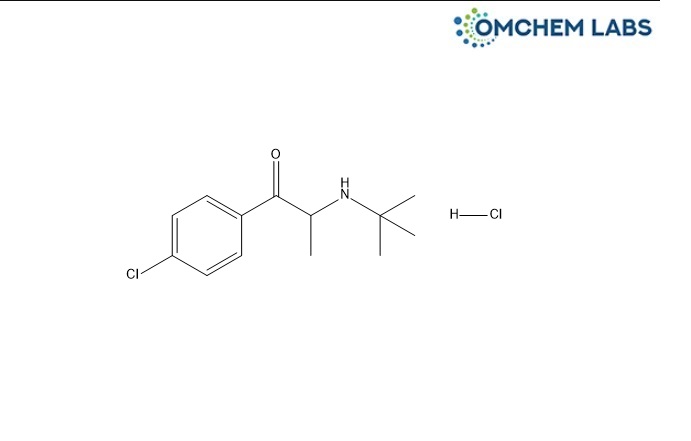
CAS No : 1049718-72-0
2-(t-Butylamino)-4'-chloropropiophenone hydrochloride 2-(tert-Butylamino)-1-(4-chlorophenyl)propan-1-one hydrochloride
CAS No : 1049718-43-5
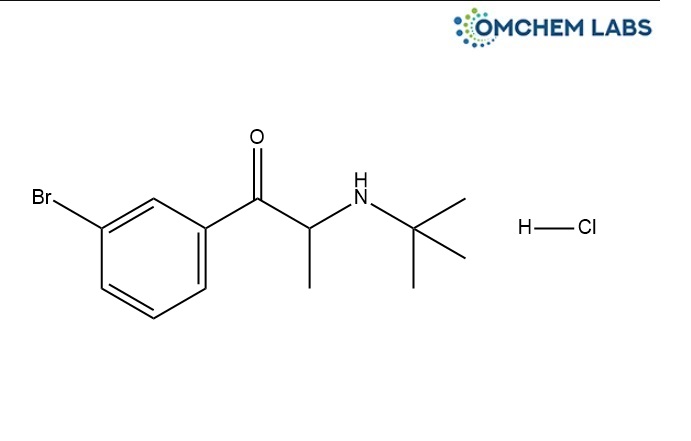
2-(tert-Butylamino)-3'-bromopropiophenone hydrochloride 2-(tert-Butylamino)-1-(3-bromophenyl)propan-1-one hydrochloride
CAS No : 152943-33-4
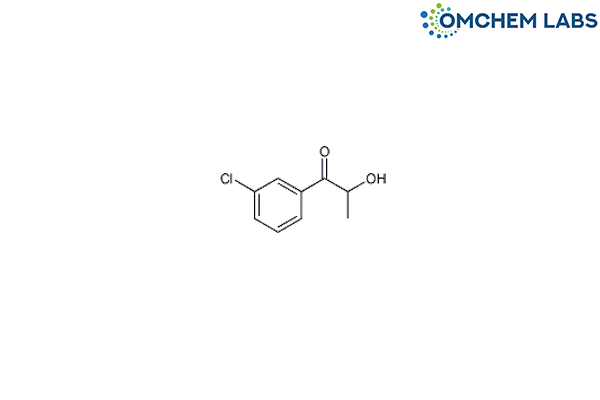
1-(3-Chlorophenyl)-2-hydroxy-1-propanone
CAS No : 10557-17-2
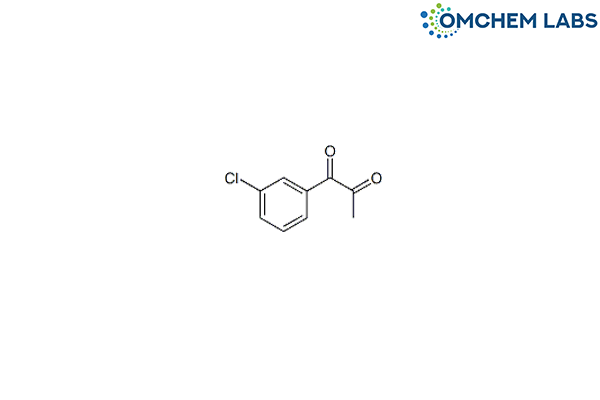
1-(3-Chlorophenyl)-1,2-propanedione
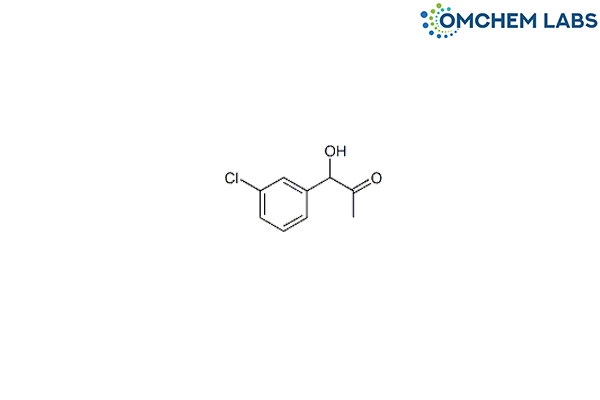
CAS No : 857233-13-7
1-(3-Chlorophenyl)-1-hydroxy-2-propanone
