Hydroxychloroquine is an antimalarial and immunomodulatory drug commonly used to treat autoimmune conditions such as rheumatoid arthritis and systemic lupus erythematosus. It works by interfering with the communication of immune cells, thereby reducing inflammation and immune response. Ensuring the pharmaceutical purity of Hydroxychloroquine is essential for maintaining its safety, therapeutic effectiveness, and adherence to regulatory standards. Impurity profiling is crucial to detect and control any unwanted substances that may arise during manufacturing, storage, or degradation. Here are some of its known impurities listed below.
Showing 1 - 5 of 5 products
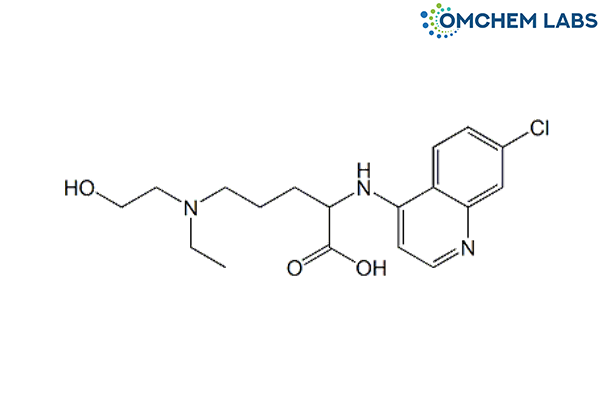
Catalogue No : HYDO-OCL-001
CAS No : 645406-24-2
In Stock
Synonyms
2-((7-Chloroquinolin-4-yl)amino)-5-(ethyl(2-hydroxyethyl)amino)pentanoic acid
2-((7-Chloroquinolin-4-yl)amino)-5-(ethyl(2-hydroxyethyl)amino)pentanoic acid

Catalogue No : HYDO-OCL-002
CAS No : 69559-11-1
In Stock
Synonyms
2-((4-Aminopentyl)(ethyl)amino)ethanol
2-((4-Aminopentyl)(ethyl)amino)ethanol
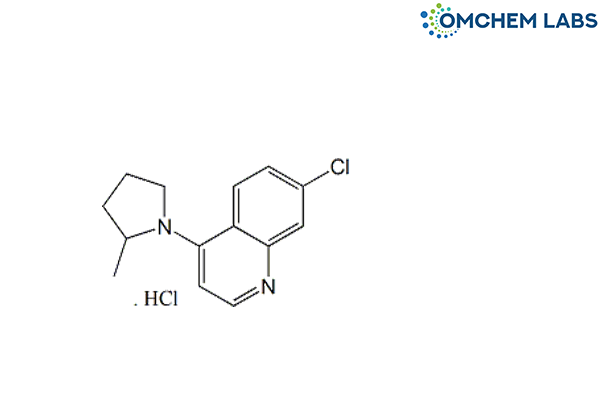
Catalogue No : HYDO-OCL-003
CAS No : 6281-58-9
In Stock
Synonyms
7-Chloro-4-[(2RS)-2-methylpyrrolidin-1-yl]quinoline hydrochloride
7-Chloro-4-[(2RS)-2-methylpyrrolidin-1-yl]quinoline hydrochloride
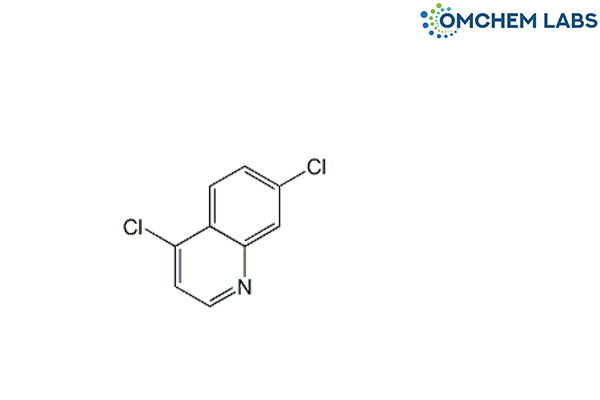
Catalogue No : HYDO-OCL-004
CAS No : 86-98-6
In Stock
Synonyms
4,7-Dichloroquinoline
4,7-Dichloroquinoline
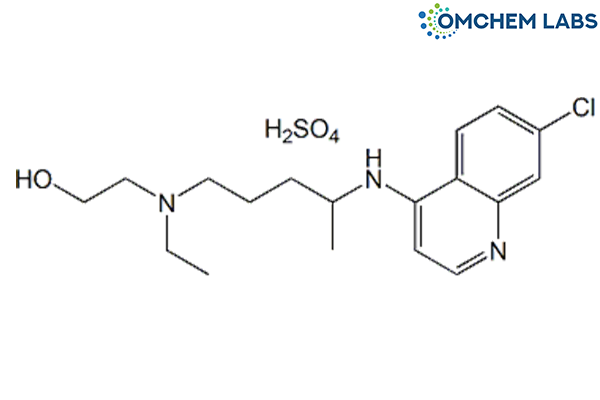
Catalogue No : HYDR-OCL-007
CAS No : 747-36-4
In Stock
Synonyms
2-[[4-[(7-Chloro-4-quinolinyl)amino]pentyl]ethylamino]ethanol sulfate
2-[[4-[(7-Chloro-4-quinolinyl)amino]pentyl]ethylamino]ethanol sulfate
