Lisinopril is an angiotensin-converting enzyme (ACE) inhibitor widely prescribed for the management of hypertension, heart failure, and post-myocardial infarction care. It works by relaxing blood vessels, thereby improving blood flow and reducing blood pressure. Maintaining the pharmaceutical purity of Lisinopril is vital to ensure consistent therapeutic performance, patient safety, and adherence to regulatory standards. Impurity profiling is essential for detecting and controlling unwanted substances that may arise during manufacturing, storage, or degradation. Here are some of its known impurities listed below.
Showing 1 - 3 of 3 products
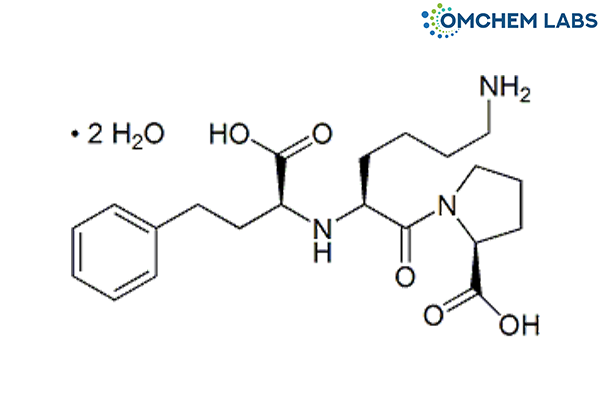
Catalogue No : LISI-OCL-001
CAS No : 83915-83-7
In Stock
Synonyms
(2S)-1-[(2S)-6-Amino-2-[[(1S)-1-carboxy-3-phenylpropyl]amino]hexanoyl]pyrrolidine-2-carboxylic acid dihydrate
(2S)-1-[(2S)-6-Amino-2-[[(1S)-1-carboxy-3-phenylpropyl]amino]hexanoyl]pyrrolidine-2-carboxylic acid dihydrate

Catalogue No : LISI-OCL-002
CAS No : 1012-05-1
In Stock
Synonyms
(2RS)-2-Amino-4-phenylbutanoic acid
(2RS)-2-Amino-4-phenylbutanoic acid
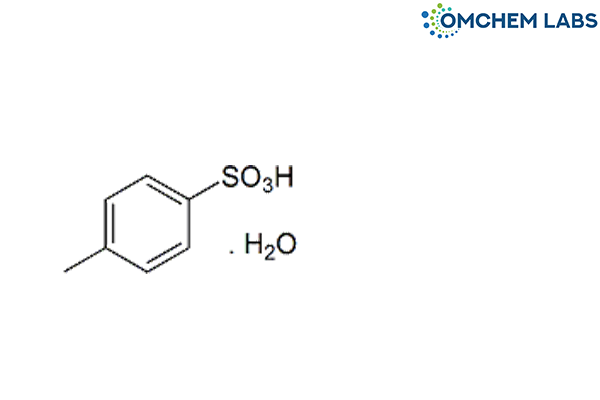
Catalogue No : LISI-OCL-003
CAS No : 6192-52-5
In Stock
Synonyms
4-Methylbenzenesulphonic acid monohydrate
4-Methylbenzenesulphonic acid monohydrate
