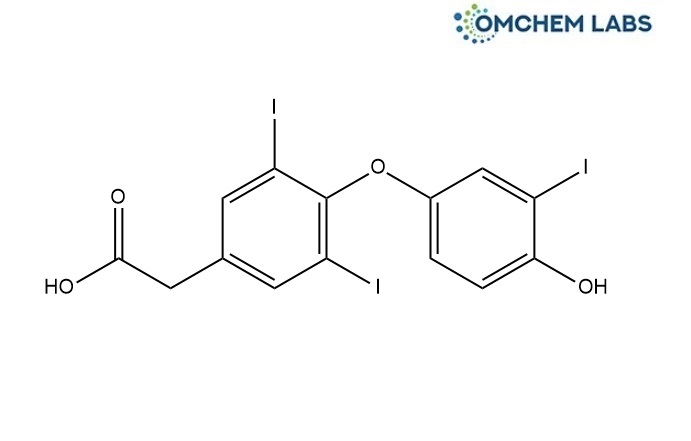
Levothyroxine Impurity C
| Catalogue No |
LEVO-OCL-013 |
| CAS NO |
51-24-1 |
| Molecular Formula | C14H9I3O4 |
| Molecular weight | 621.93 |
| Inquiry Status | In Stock |
| Synonyms | [4-(4-Hydroxy-3-iodophenoxy)-3,5-diiodophenyl]acetic Acid; 2-(4-(4-Hydroxy-3-iodophenoxy)-3,5-diiodophenyl)acetic Acid; 4-(4-Hydroxy-3-iodophenoxy)-3,5-diiodobenzeneacetic Acid; |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Levothyroxine Impurity C: A Scientific Perspective
Introduction
The consistent quality of pharmaceutical products is critically dependent on thorough impurity profiling of their active pharmaceutical ingredients (APIs). Levothyroxine Impurity C represents one of several trace-level substances that may emerge during the manufacturing or storage of levothyroxine. Impurities such as this must be precisely identified, monitored, and controlled, as they may affect the safety, efficacy, or stability of the drug. Comprehensive profiling ensures regulatory compliance and safeguards therapeutic performance throughout the product's lifecycle.
Formation of Impurities During API Synthesis
Impurities like Levothyroxine Impurity C typically originate from complex reaction pathways or degradation processes inherent to API synthesis. These unwanted entities may arise from incomplete reactions, secondary chemical pathways, interactions with residual solvents or reagents, or environmental factors such as moisture or light. In some instances, even the quality of raw materials or reaction intermediates contributes to impurity formation. These processes often yield structurally related substances that must be isolated and understood to maintain process integrity and reproducibility.
Analytical Data Interpretation Techniques
Uncovering and interpreting the impurity landscape within a pharmaceutical substance requires the integration of modern analytical technologies. Techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and hybrid systems like LC-MS provide vital resolution and specificity. For compounds like Levothyroxine Impurity C, spectral analysis via mass spectrometry or nuclear magnetic resonance (NMR) supports elucidation of structural identity and helps determine the relationship between impurity and parent compound. Data interpretation must be rigorous, as subtle variations in molecular structure may significantly influence biological activity or safety.
Method Validation for Impurity Detection
Analytical methods employed for impurity detection must be subjected to comprehensive validation to ensure reliability and precision. Regulatory standards require validated procedures that demonstrate accuracy, specificity, reproducibility, and sensitivity. For impurities associated with levothyroxine, this ensures that even trace-level compounds like Impurity C are consistently detected and quantified within acceptable limits. Method validation also forms the basis for global regulatory submissions and is essential in establishing product quality specifications.
Purification Strategies for Reducing Impurities
The control of impurities during and after synthesis often involves targeted purification techniques. In the case of Levothyroxine Impurity C, purification may be approached through crystallization, solvent extraction, or chromatographic separation. These methods are selected based on the impurity's physicochemical properties in relation to the API. A well-optimized purification protocol not only reduces impurity content to acceptable thresholds but also enhances the stability and performance of the final drug substance.
Isolation and Characterization of Impurities
When impurities are present at levels requiring identification or toxicological assessment, they must be isolated and structurally characterized. For Levothyroxine Impurity C, preparative-scale chromatographic methods are often employed for isolation, followed by spectral techniques for structural elucidation. Understanding the nature of such impurities is essential for risk assessment and for developing appropriate impurity limits. In some cases, reference standards are synthesized or isolated to aid in ongoing quality control.
Conclusion
The impurity profiling of Levothyroxine Impurity C is a critical scientific process that integrates synthetic chemistry, advanced analytics, and regulatory insight. Through comprehensive detection, characterization, validation, and control strategies, pharmaceutical developers can ensure the purity, safety, and efficacy of levothyroxine-based products. Establishing a proactive and robust impurity management system is key to maintaining high standards in drug development and aligning with evolving regulatory expectations.
