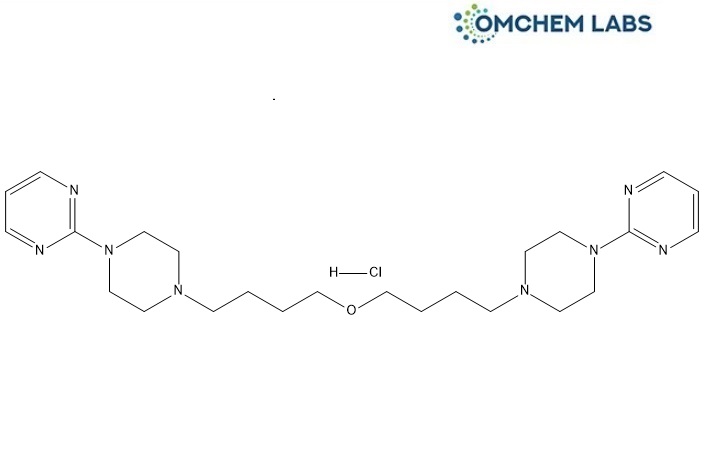
Buspirone Impurity D (HCL)
| Catalogue No |
BUSP-OCL-004 |
| CAS NO |
N/A |
| Molecular Formula | C24H39ClN8O |
| Molecular weight | 491.07 |
| Inquiry Status | In Stock |
| Synonyms | 2,2`-[Oxybis[butane-1,4-diyl(piperazine-1,4-diyl)]]dipyrimidine hydrochloride |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Buspirone Impurity D (HCl): A Scientific Perspective
Introduction
The presence and evaluation of impurities remain a cornerstone of pharmaceutical quality control, as they directly influence the safety and therapeutic reliability of drug substances. In the case of Buspirone Impurity D (HCl), profiling plays a pivotal role in ensuring that the associated active pharmaceutical ingredient (API) is both effective and compliant with international regulatory standards. Impurity profiling encompasses the systematic detection, quantification, characterization, and control of extraneous chemical entities that may arise during synthesis or subsequent handling. A holistic approach to impurity management is essential for safeguarding product integrity throughout the drug development process and lifecycle.
Formation of Impurities During API Synthesis
Impurities in Buspirone Impurity D (HCl) may be generated at multiple stages of its synthesis. Synthetic pathways often involve complex chemical reactions, each with the potential to yield unintended by-products or incomplete intermediates. Residual reagents, catalysts, and solvents may persist if not adequately removed, contributing to the impurity profile. Additionally, environmental factors such as light exposure, oxidative conditions, or variations in processing parameters may accelerate degradation pathways. Understanding these mechanisms allows researchers and manufacturers to anticipate impurity formation and design strategies to limit their presence in the final material.
Analytical Data Interpretation Techniques
Accurate impurity profiling depends heavily on advanced analytical methodologies. For Buspirone Impurity D (HCl), chromatographic and spectroscopic tools form the backbone of impurity detection and interpretation. Techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and hyphenated methods like LC-MS are widely used to separate and identify minor constituents within complex mixtures. Complementary techniques such as nuclear magnetic resonance (NMR) or infrared spectroscopy (IR) may further elucidate structural characteristics. Beyond mere detection, data interpretation focuses on recognizing patterns, resolving overlapping peaks, and distinguishing genuine impurities from analytical artifacts, ensuring that results provide a trustworthy reflection of the impurity landscape.
Method Validation for Impurity Detection
For analytical data to hold regulatory credibility, the employed methods must undergo rigorous validation. Validation of techniques applied to Buspirone Impurity D (HCl) is designed to demonstrate that analytical systems are reliable, reproducible, and robust under varied conditions. Validation parameters typically address specificity, linearity, sensitivity, and precision, ensuring that even trace-level impurities can be consistently identified and quantified. A validated method forms the foundation for regulatory submissions, as it assures health authorities that the impurity profile has been established using scientifically sound and internationally recognized procedures.
Purification Strategies for Reducing Impurities
Purification serves as a practical measure to minimize impurity levels in Buspirone Impurity D (HCl) during or after synthesis. Different strategies may be applied depending on the chemical properties of the compound and its associated impurities. Crystallization offers a simple yet effective approach, exploiting solubility differences to exclude undesired entities. In contrast, more refined approaches, such as chromatographic purification or selective solvent extraction, provide tailored separation when impurities are structurally similar to the desired compound. Distillation may also be relevant for volatile components. Selecting an appropriate purification method requires balancing impurity removal efficiency with yield and scalability considerations.
Isolation and Characterization of Impurities
Isolation of impurities from Buspirone Impurity D (HCl) is critical for full structural characterization and toxicological assessment. Techniques such as preparative chromatography or large-scale crystallization may be employed to obtain sufficient quantities of an impurity for detailed study. Once isolated, advanced structural elucidation tools, including NMR, MS, and IR spectroscopy, provide the necessary resolution to define the molecular framework. Characterization of impurities is not only a regulatory requirement but also an important step in understanding potential safety risks, enabling the establishment of scientifically justified acceptance criteria.
Conclusion
The impurity profiling of Buspirone Impurity D (HCl) represents a multidimensional process, integrating knowledge of synthetic chemistry, analytical science, and regulatory compliance. From the identification of impurity-generating mechanisms to the application of validated analytical techniques, every stage contributes to a comprehensive understanding of impurity behavior. Purification and isolation further ensure that impurities are managed effectively, supporting the safety, efficacy, and consistency of the final pharmaceutical product. A robust impurity control strategy for Buspirone Impurity D (HCl) thus underpins both patient safety and the overall quality framework demanded in the modern pharmaceutical industry.
