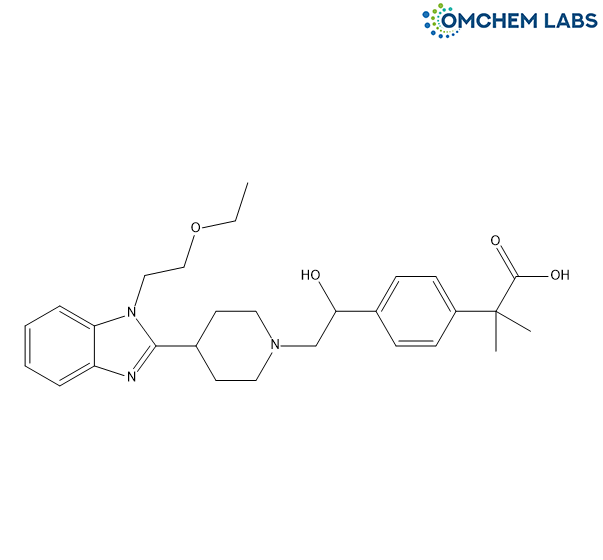
1’-Hydroxy Bilastine
| Catalogue No |
BILA-OCL-011 |
| CAS NO |
1638785-23-5 |
| Molecular Formula | C28H37N3O4 |
| Molecular weight | 479.62 |
| Inquiry Status | In Stock |
| Synonyms | 2-(4-(2-(4-(1-(2-Ethoxyethyl)-1H-benzo[d]imidazol-2-yl)piperidin-1-yl)-1-hydroxyethyl)phenyl)-2-methylpropanoic Acid |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of 1’-Hydroxy Bilastine: A Scientific Overview
Introduction
The presence and evaluation of impurities within pharmaceutical substances is a fundamental aspect of drug quality and regulatory compliance. In the case of 1’-Hydroxy Bilastine, impurity profiling provides insights into the origin, structural identity, and potential risks associated with undesired components formed during its synthesis or storage. Understanding impurities is not only a matter of meeting pharmacopeial requirements but also of ensuring that the final product maintains consistent safety and therapeutic performance. A comprehensive impurity study involves multiple layers, including synthesis knowledge, analytical assessment, method validation, purification measures, and characterization strategies.
Formation of Impurities During API Synthesis
Impurities in 1’-Hydroxy Bilastine may emerge from various stages of its synthetic route. Raw material residues, incomplete reactions, side products, and degradation pathways all contribute to impurity generation. Process conditions such as temperature, solvent systems, catalyst use, and reaction time influence the scale and type of impurities formed. Additionally, environmental factors—such as exposure to oxygen, light, or moisture—may lead to degradation impurities, further complicating the impurity profile. A strong grasp of the chemistry behind the synthesis pathway is therefore essential for predicting and minimizing impurity formation.
Analytical Data Interpretation Techniques
Accurate impurity profiling depends on advanced analytical methodologies designed to both detect and characterize impurities associated with 1’-Hydroxy Bilastine. High-performance liquid chromatography (HPLC), gas chromatography (GC), and hyphenated techniques like LC-MS and LC-NMR are commonly applied to separate, identify, and quantify impurities. These tools provide detailed chromatographic and spectral data, enabling differentiation between structurally similar compounds. Interpretation of such data involves assessing retention behavior, spectral signatures, and fragmentation patterns, thereby building a clear picture of the impurity landscape. The integration of multiple analytical methods ensures both sensitivity and reliability in impurity identification.
Method Validation for Impurity Detection
Analytical techniques used for profiling impurities in 1’-Hydroxy Bilastine must be rigorously validated to guarantee accuracy and reproducibility. International guidelines such as ICH Q2(R1) emphasize validation parameters including specificity, precision, linearity, robustness, and detection capability. Validation ensures that methods remain consistent across laboratories and production batches, allowing regulators and manufacturers to have confidence in the impurity data generated. A validated method is not only a regulatory requirement but also a cornerstone of scientific credibility in pharmaceutical quality control.
Purification Strategies for Reducing Impurities
Purification plays an indispensable role in limiting the impurity burden in 1’-Hydroxy Bilastine. Techniques are selected based on the physicochemical properties of the target compound and its associated impurities. Crystallization may be applied to exploit solubility differences, while solvent extraction and distillation serve as effective tools for removing volatile or partitionable contaminants. Chromatographic separation, particularly preparative-scale chromatography, offers high selectivity for structurally similar impurities. A carefully designed purification protocol not only improves the overall purity of the active substance but also enhances process efficiency and consistency.
Isolation and Characterization of Impurities
When impurities exceed reporting thresholds or remain unidentified, they must be isolated and characterized in detail. For 1’-Hydroxy Bilastine, isolation is generally achieved using preparative chromatographic methods capable of separating low-level impurities from the bulk compound. Once isolated, characterization is carried out using structural elucidation techniques such as nuclear magnetic resonance (NMR), mass spectrometry (MS), and infrared (IR) spectroscopy. This step provides a complete understanding of the chemical identity and potential toxicological impact of impurities, which is vital for risk assessment and regulatory documentation.
Conclusion
The impurity profiling of 1’-Hydroxy Bilastine represents a multifaceted scientific undertaking that encompasses synthesis knowledge, analytical expertise, validation rigor, purification efficiency, and structural elucidation. Each stage is critical for achieving a high level of quality assurance and regulatory compliance. By adopting a systematic approach to impurity detection, isolation, and characterization, manufacturers can ensure that 1’-Hydroxy Bilastine meets international safety standards while maintaining therapeutic reliability. Ultimately, impurity profiling serves as both a regulatory requirement and a safeguard for patient well-being.
