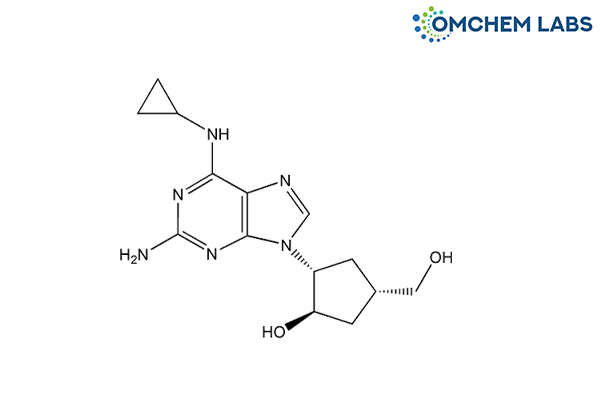
Abacavir 2-Hydroxy Impurity
| Catalogue No |
ABAC-OCL-002 |
| CAS NO |
NA |
| Molecular Formula | C14H20N6O2 |
| Molecular weight | 304.35 |
| Inquiry Status | In Stock |
| Synonyms | (1R,2R,4S)-2-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]-4-(hydroxy methyl)cyclopentan-1-ol |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Abacavir 2-Hydroxy Impurity: A Scientific Perspective
Introduction
In the realm of pharmaceutical development, the presence and control of impurities are critical to ensuring product quality, therapeutic consistency, and patient safety. Abacavir 2-Hydroxy Impurity, as a trace-level constituent arising during active pharmaceutical ingredient (API) production, plays a key role in overall impurity profiling strategies. The ability to thoroughly assess such structurally related impurities is central to maintaining regulatory compliance and process integrity. While minor in concentration, such impurities may impact stability, pharmacokinetics, or toxicity, warranting a systematic and science-driven approach to their identification and control.
Formation of Impurities During API Synthesis
The generation of impurities like Abacavir 2-Hydroxy Impurity can stem from various mechanistic pathways within the synthetic sequence. These include incomplete conversions of intermediates, over-reaction of functional groups, side reactions from reagents or catalysts, and degradation of sensitive moieties under thermal, oxidative, or hydrolytic stress. In certain synthetic workflows, residual solvents or reagents may further interact with intermediate species to form structurally modified impurities. Such formation mechanisms are often highly dependent on process conditions and reaction environment, making early-stage process understanding essential for anticipating impurity outcomes.
Analytical Data Interpretation Techniques
The detection and structural elucidation of impurities demand a robust and multi-instrumental analytical framework. Profiling Abacavir 2-Hydroxy Impurity typically involves techniques like high-performance liquid chromatography (HPLC), ultra-performance liquid chromatography (UPLC), gas chromatography (GC), and hyphenated systems such as LC-MS and LC-NMR. Each method provides distinct insights—ranging from retention characteristics to mass-to-charge ratios and molecular fragmentation patterns. An integrated interpretation of chromatographic peaks and spectral data enables a comprehensive understanding of impurity identity, behavior, and possible origin. Modern software-assisted analysis further aids in resolving co-eluting species and unknown components within complex matrices.
Method Validation for Impurity Detection
Ensuring the reliability of impurity testing requires method validation aligned with current regulatory expectations. For impurities like Abacavir 2-Hydroxy Impurity, the analytical approach must be rigorously assessed for parameters such as selectivity, accuracy, precision, linearity, and detection thresholds. This not only builds confidence in the analytical method’s capability but also ensures that the impurity can be reproducibly identified and quantified under routine conditions. Validation protocols are designed to demonstrate method robustness and suitability across a range of expected operating conditions and sample variations.
Purification Strategies for Reducing Impurities
Once detected, efforts are made to reduce or eliminate impurities through optimized purification steps within the manufacturing process. Depending on the chemical nature and polarity differences of Abacavir 2-Hydroxy Impurity, several purification strategies may be employed. Techniques such as recrystallization, liquid-liquid extraction, flash chromatography, and selective precipitation are commonly adapted to separate impurities from the desired API. In process development, continuous purification optimization helps achieve consistent impurity clearance while maintaining desirable product yield and physicochemical properties.
Isolation and Characterization of Impurities
When an impurity is present above established identification thresholds or if its structural identity remains unknown, it becomes necessary to isolate and characterize it using advanced methodologies. For Abacavir 2-Hydroxy Impurity, preparative chromatographic techniques may be employed to obtain material in sufficient purity for structural analysis. Once isolated, detailed studies using techniques such as nuclear magnetic resonance (NMR), high-resolution mass spectrometry (HRMS), and infrared (IR) spectroscopy are performed to determine molecular configuration and functional group distribution. This enables accurate classification of the impurity and supports regulatory justification for its control limits.
Conclusion
The impurity profiling of Abacavir 2-Hydroxy Impurity represents a multi-faceted scientific endeavor encompassing synthesis knowledge, analytical expertise, validation strategy, and chemical characterization. Addressing such impurities is vital not only for meeting pharmacopoeial guidelines but also for reinforcing the overall safety and quality framework of pharmaceutical production. A proactive and methodical approach to impurity profiling ultimately contributes to the development of a robust API manufacturing process and a safe, effective drug product.
