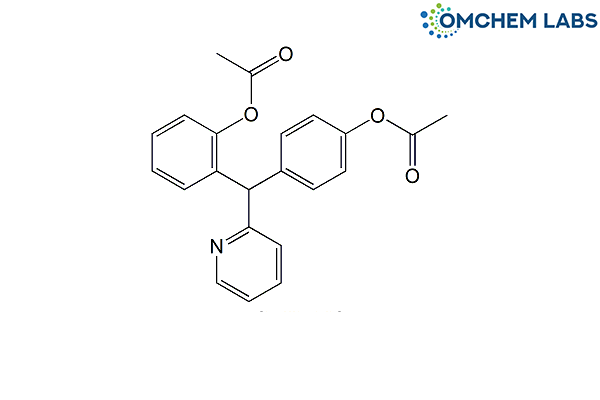
Bisacodyl Impurity E
| Catalogue No |
BISA-OCL-005 |
| CAS NO |
111664-35-8 |
| Molecular Formula | C22H19NO4 |
| Molecular weight | 361.39 |
| Inquiry Status | In Stock |
| Synonyms | 2-[(RS)-[4-(Acetyloxy)-phenyl](pyridin-2-yl)methyl]phenyl acetate |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Bisacodyl Impurity E: A Strategic Approach to Quality Control in API Manufacturing
Introduction
In the pharmaceutical sector, impurity profiling of active pharmaceutical ingredients (APIs) plays a pivotal role in ensuring therapeutic safety and compliance with regulatory standards. Bisacodyl Impurity E serves as a representative example of how structurally or process-related impurities demand thorough scientific investigation during drug development. Profiling such impurities helps prevent undesired pharmacological effects, enhances product stability, and ensures consistent quality throughout the production lifecycle. Understanding the nature and control of impurities is essential for building a comprehensive quality framework around any pharmaceutical compound.
Formation of Impurities During API Synthesis
The synthesis of APIs often involves complex chemical reactions, each with the potential to introduce unwanted molecular species. In the case of Bisacodyl Impurity E, impurities may form due to incomplete reactions, degradation of intermediates, or side reactions influenced by factors such as temperature, solvent polarity, or catalyst behavior. Additionally, external conditions like light exposure or residual moisture can accelerate impurity development during post-synthesis handling or storage. These impurities, while sometimes present in trace amounts, require detailed profiling to prevent accumulation or interaction within the final dosage form.
Analytical Data Interpretation Techniques
Modern impurity profiling relies heavily on analytical science to reveal the chemical signatures of undesired compounds. For Bisacodyl Impurity E, advanced methodologies such as high-performance liquid chromatography (HPLC), liquid chromatography–mass spectrometry (LC-MS), gas chromatography (GC), and nuclear magnetic resonance (NMR) spectroscopy are typically used to obtain clear insights into impurity composition. By interpreting spectral and chromatographic outputs, scientists can evaluate peak purity, retention patterns, and fragmentation profiles. The goal is not only to identify known impurities but also to detect any unexpected or novel variants that could compromise quality or safety.
Method Validation for Impurity Detection
To support reliable impurity detection, analytical methods must undergo rigorous validation in alignment with global regulatory frameworks. The validation process for methods used in the study of Bisacodyl Impurity E typically includes testing for specificity, accuracy, precision, linearity, and robustness. Validation ensures that even low-level impurities can be consistently detected and quantified with reproducible results. It also establishes confidence in the analytical tools being used for batch release testing, stability studies, and process verification, which are crucial in regulated environments.
Purification Strategies for Reducing Impurities
Effective purification is essential for minimizing impurity levels in the final API. In the context of Bisacodyl Impurity E, a variety of techniques can be employed depending on the physicochemical behavior of the impurity relative to the main compound. Common approaches include recrystallization, solvent extraction, column chromatography, and phase separation. These methods aim to exploit differences in solubility, polarity, or boiling point to selectively isolate the API from undesired components. Process optimization during purification also contributes to improved yield, reduced waste, and greater product consistency.
Isolation and Characterization of Impurities
When impurity levels exceed defined limits or when a structure remains unidentified, isolation becomes necessary for further study. For Bisacodyl Impurity E, isolation might involve preparative chromatographic methods followed by detailed spectroscopic analysis. Characterization often employs techniques such as NMR, MS, and IR spectroscopy to confirm structural identity and assess potential pharmacological or toxicological relevance. These findings are then used to define impurity acceptance criteria and, when applicable, establish reference standards for routine analysis.
Conclusion
The comprehensive profiling of Bisacodyl Impurity E exemplifies the scientific rigor required to manage impurity-related risks in pharmaceutical development. From synthetic origin to analytical interpretation, validation, purification, and structural elucidation, each phase contributes to a well-rounded impurity control strategy. Such an approach not only enhances product quality and regulatory compliance but also upholds patient safety, which remains the central tenet of pharmaceutical science. Robust impurity profiling practices continue to evolve, supporting more precise and predictive quality control in modern drug manufacturing.
