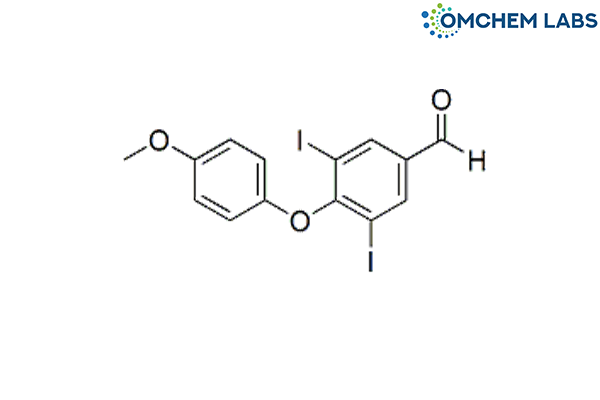
Levothyroxine Methoxyphenoxy Aldehyde Impurity
| Catalogue No |
LEVO-OCL-003 |
| CAS NO |
69240-57-9 |
| Molecular Formula | C14H10I2O3 |
| Molecular weight | 480.04 |
| Inquiry Status | In Stock |
| Synonyms | 4-(4-Methoxyphenoxy)-3,5-diiodobenzaldehyde |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Levothyroxine Methoxyphenoxy Aldehyde Impurity
Introduction
Pharmaceutical impurity profiling is a cornerstone of drug substance development, ensuring the safety, efficacy, and regulatory compliance of active pharmaceutical ingredients (APIs). In the case of Levothyroxine Methoxyphenoxy Aldehyde Impurity, comprehensive understanding of its origin, detection, and control strategies is essential. Impurities like this can influence the therapeutic profile of the final product and, if not properly addressed, may compromise patient safety or lead to non-compliance with international quality standards. The purpose of impurity profiling is not merely identification but also systematic evaluation and control throughout the product lifecycle.
Formation of Impurities During API Synthesis
Impurities such as Levothyroxine Methoxyphenoxy Aldehyde Impurity can be introduced during multiple stages of synthesis. They may result from incomplete reactions, interaction of reagents, degradation of intermediates, or unintended side reactions under specific processing conditions. Factors such as solvent compatibility, catalyst behavior, reaction pH, temperature range, and oxidative stress can all contribute to impurity generation. Additionally, post-synthesis processing—such as drying, crystallization, or prolonged storage—can create or amplify impurity presence. Understanding these pathways is vital for risk mitigation and refining synthetic protocols to minimize impurity carryover.
Analytical Data Interpretation Techniques
Detecting and profiling impurities require a suite of advanced analytical tools that offer both sensitivity and specificity. For Levothyroxine Methoxyphenoxy Aldehyde Impurity, techniques such as high-performance liquid chromatography (HPLC), ultra-performance liquid chromatography (UPLC), gas chromatography (GC), and mass spectrometry (MS) are typically employed. Nuclear magnetic resonance (NMR) spectroscopy and infrared (IR) spectroscopy provide additional structural insights. The interpretation of chromatographic and spectroscopic data involves the identification of retention patterns, fragmentation behavior, and spectral signatures. These analyses collectively help isolate known impurities and flag potential unknowns for further investigation.
Method Validation for Impurity Detection
To ensure reliability in detecting and quantifying Levothyroxine Methoxyphenoxy Aldehyde Impurity, analytical methods must undergo stringent validation. Validation confirms that the chosen methods are suitable for their intended purpose and can consistently deliver accurate results under varied conditions. Parameters such as specificity, accuracy, precision, robustness, linearity, and sensitivity (limit of detection and quantification) are critically evaluated. A validated method strengthens the integrity of impurity data and is a regulatory necessity when submitting drug applications or changes to health authorities.
Purification Strategies for Reducing Impurities
Controlling impurity levels involves applying targeted purification approaches tailored to the physicochemical properties of both the API and its associated impurities. In the context of Levothyroxine Methoxyphenoxy Aldehyde Impurity, methods such as recrystallization, solvent partitioning, activated carbon treatment, and column chromatography are routinely considered. Each technique offers unique advantages depending on solubility, polarity, thermal stability, and molecular weight. By optimizing purification parameters, manufacturers can significantly enhance product purity while maintaining process efficiency and yield.
Isolation and Characterization of Impurities
When impurities exceed acceptable thresholds or remain structurally undefined, isolation becomes necessary. For impurities like Levothyroxine Methoxyphenoxy Aldehyde Impurity, preparative chromatography often plays a key role in obtaining purified samples. Subsequent characterization is performed using high-resolution techniques such as NMR, MS, and UV-Vis spectroscopy. Structural elucidation not only helps in confirming the impurity’s identity but also supports toxicological evaluations and reference standard development. Accurate characterization forms the backbone of a robust impurity control strategy and is critical for long-term product quality assurance.
Conclusion
The impurity profiling of Levothyroxine Methoxyphenoxy Aldehyde Impurity underscores the complex interplay between synthetic chemistry, analytical science, and regulatory expectations. From its potential formation in synthetic routes to its detection, validation, purification, and eventual structural definition, each step demands precision and scientific rigor. A well-structured impurity profiling strategy contributes not only to regulatory compliance but also to the overall safety and effectiveness of pharmaceutical therapies. Establishing proactive impurity control measures ensures that the API remains consistent, reliable, and safe across every batch and formulation cycle.
