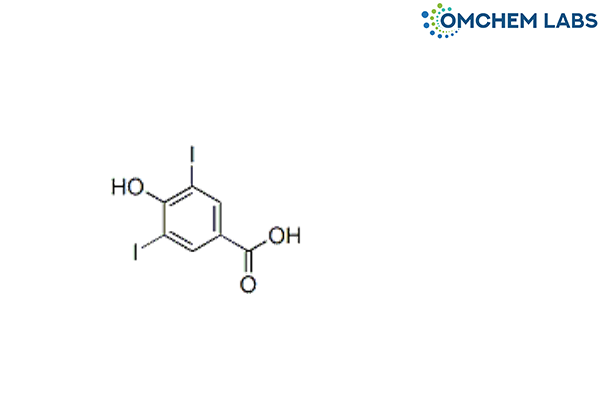
Levothyroxine 3,5-Diiodo 4-Hydroxybenzoic Acid
| Catalogue No |
LEVO-OCL-004 |
| CAS NO |
618-76-8 |
| Molecular Formula | C7H4I2O3 |
| Molecular weight | 389.91 |
| Inquiry Status | In Stock |
| Synonyms | 4-Hydroxy-3,5-diiodobenzoic acid |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Levothyroxine 3,5-Diiodo 4-Hydroxybenzoic Acid: A Comprehensive Scientific Perspective
Introduction
In modern pharmaceutical manufacturing, the impurity profiling of related substances plays a critical role in safeguarding the therapeutic efficacy and safety of active pharmaceutical ingredients (APIs). One such substance of importance is Levothyroxine 3,5-Diiodo 4-Hydroxybenzoic Acid, which can be present as a minor impurity during the synthesis of thyroid hormone analogs. Profiling this impurity requires a multidimensional approach, combining synthetic pathway understanding, robust analytical science, and validated purification strategies. The goal is not only to ensure regulatory compliance but also to maintain patient safety by establishing comprehensive impurity control mechanisms.
Formation of Impurities During API Synthesis
The emergence of Levothyroxine 3,5-Diiodo 4-Hydroxybenzoic Acid during the synthesis of levothyroxine or structurally related thyroid APIs is primarily attributed to partial iodination, oxidative stress reactions, and residual synthetic intermediates. Such impurities may develop through incomplete reactions, excess iodinating agents, or secondary degradation under light, temperature, or pH fluctuations. Furthermore, reaction side chains and the presence of trace-level reagents or catalysts can contribute to structural variants that manifest as related impurities. Understanding these chemical transformations is essential for establishing a reliable impurity control strategy from the earliest stages of process development.
Analytical Data Interpretation Techniques
Accurate identification and differentiation of Levothyroxine 3,5-Diiodo 4-Hydroxybenzoic Acid from the main API and other structurally similar compounds require the integration of advanced analytical techniques. Chromatographic methods, such as high-performance liquid chromatography (HPLC) and ultra-performance liquid chromatography (UPLC), serve as the primary tools for separating and detecting trace-level impurities. These are often coupled with mass spectrometry (MS) or nuclear magnetic resonance (NMR) to confirm molecular identity and structural orientation. Sophisticated data interpretation models aid in deconvoluting overlapping peaks, validating retention characteristics, and establishing impurity fingerprints that are reproducible and reliable for quality assurance purposes.
Method Validation for Impurity Detection
To ensure the consistency and credibility of analytical results, method validation is an essential requirement. Analytical methods tailored for the detection of Levothyroxine 3,5-Diiodo 4-Hydroxybenzoic Acid must undergo rigorous qualification in accordance with international guidelines such as ICH Q2(R1). This includes evaluating parameters such as selectivity, linearity, accuracy, precision, and detection limits. Only through a validated procedure can the impurity be consistently quantified with confidence, allowing its inclusion in specifications and regulatory dossiers. Method robustness also plays a crucial role in ensuring reproducibility across different laboratories and manufacturing sites.
Purification Strategies for Reducing Impurities
Post-synthesis purification strategies are key to minimizing the levels of Levothyroxine 3,5-Diiodo 4-Hydroxybenzoic Acid in the final API. Crystallization is a frequently employed method that leverages solubility differentials to separate impurities from the desired product. In more complex cases, preparative chromatography and solvent extraction techniques are implemented to achieve higher selectivity. The purification process is typically optimized to enhance recovery while simultaneously ensuring impurity removal. The selection of an appropriate technique is guided by the physicochemical characteristics of both the API and the impurity, including polarity, thermal stability, and solubility profile.
Isolation and Characterization of Impurities
In cases where the impurity exceeds qualification thresholds or remains unidentified, it becomes necessary to isolate and characterize Levothyroxine 3,5-Diiodo 4-Hydroxybenzoic Acid in its pure form. This process often begins with large-scale chromatographic isolation followed by comprehensive structural elucidation using MS, NMR, and infrared (IR) spectroscopy. Structural knowledge facilitates toxicological assessment and helps in determining whether the impurity has any pharmacodynamic or toxicological relevance. Once characterized, such impurities may also serve as reference materials for future batch comparisons and stability studies.
Conclusion
The systematic profiling of Levothyroxine 3,5-Diiodo 4-Hydroxybenzoic Acid represents a vital component of pharmaceutical quality control. From its origin in synthesis to its elimination through purification, every stage must be guided by rigorous scientific methodology and a deep understanding of chemical behavior. Advanced analytical tools, supported by validated protocols and structural characterization techniques, provide a reliable framework for controlling such impurities. Ensuring a low and controlled presence of this impurity enhances the overall quality and safety of the API, supports regulatory compliance, and upholds patient trust in pharmaceutical therapies.
