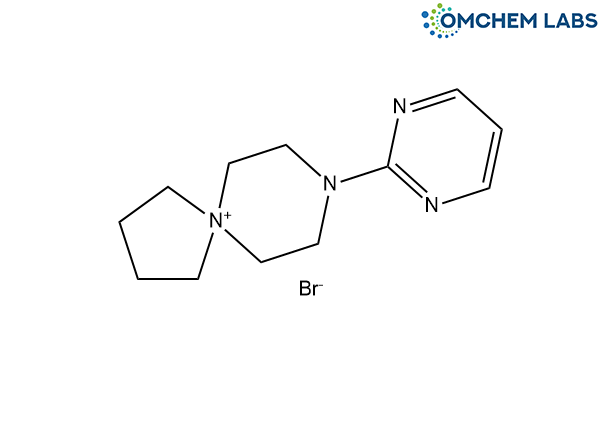
Buspirone Impurity B
| Catalogue No |
BUSP-OCL-002 |
| CAS NO |
81461-73-6 |
| Molecular Formula | C12H19N4 : Br |
| Molecular weight | 299.21 |
| Inquiry Status | In Stock |
| Synonyms | 8-(Pyrimidin-2-yl)-8-aza-5-azoniaspiro[4.5]decane bromide |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Buspirone Impurity B: A Scientific Perspective
Introduction
Pharmaceutical drug development demands stringent control over impurities to ensure therapeutic safety and regulatory acceptance. Among various categories of impurities, structurally related compounds often require special attention due to their potential to influence drug quality. Buspirone Impurity B represents such a case, where systematic impurity profiling provides insights into its origin, behavior, and control. The study of impurities associated with Buspirone is not merely a regulatory formality; it is a scientific exercise that enhances the understanding of synthetic processes, stability characteristics, and quality assurance strategies.
Formation of Impurities During API Synthesis
The genesis of impurities in Buspirone Impurity B can be traced to different stages of active pharmaceutical ingredient (API) synthesis. Impurities may emerge from incomplete conversions of starting materials, side reactions occurring under specific reaction conditions, or from the influence of solvents, catalysts, and intermediates. Environmental factors such as heat, light, and humidity can further contribute to degradation by-products, complicating the impurity landscape. Understanding these formation pathways is essential for designing robust synthetic processes and implementing control strategies that minimize unwanted variations.
Analytical Data Interpretation Techniques
Robust analytical characterization is the cornerstone of impurity profiling. For Buspirone Impurity B, modern techniques such as high-performance liquid chromatography, mass spectrometry, nuclear magnetic resonance spectroscopy, and gas chromatography are commonly deployed to detect and interpret impurity signatures. Each technique provides complementary insights, whether in terms of molecular weight determination, structural elucidation, or chromatographic separation. Analytical interpretation involves comparing spectral data, retention behavior, and fragmentation patterns to build a comprehensive impurity profile. This process not only aids in impurity identification but also supports long-term monitoring of production consistency.
Method Validation for Impurity Detection
Analytical procedures applied to Buspirone Impurity B must be rigorously validated to ensure reliability and reproducibility. International regulatory guidelines, such as ICH standards, outline key validation parameters including specificity, accuracy, precision, linearity, sensitivity, and robustness. Validating impurity detection methods guarantees that even trace levels of impurities can be consistently monitored, enhancing both product safety and regulatory confidence. A validated methodology forms the scientific backbone of impurity profiling, enabling laboratories to generate results that stand up to scrutiny across different environments and production scales.
Purification Strategies for Reducing Impurities
Minimizing the presence of Buspirone Impurity B within the API requires effective purification strategies tailored to the physicochemical properties of the compound and its related impurities. Common approaches include crystallization, solvent extraction, fractional distillation, and chromatographic separation. Each technique offers distinct advantages, with selection dependent on the solubility, volatility, or polarity differences between the impurity and the parent API. Strategic purification not only reduces impurity levels but also enhances the reproducibility of manufacturing outcomes, ensuring that the drug product maintains a consistent and acceptable purity profile.
Isolation and Characterization of Impurities
For meaningful toxicological and regulatory evaluation, impurities such as Buspirone Impurity B must often be isolated and fully characterized. Isolation typically employs preparative chromatographic methods that allow for the separation of impurity fractions in sufficient quantities. Once isolated, structural elucidation is performed using advanced spectroscopic tools such as NMR, MS, and IR. This characterization provides vital information about the molecular nature of the impurity, enabling the establishment of acceptable thresholds and guiding the creation of reference standards. By isolating and characterizing impurities, manufacturers demonstrate control over the impurity landscape and meet global compliance requirements.
Conclusion
The impurity profiling of Buspirone Impurity B represents a multifaceted process that spans synthesis, analysis, validation, purification, and structural elucidation. Each stage contributes to a comprehensive understanding of impurity behavior and ensures compliance with international quality standards. By integrating analytical precision with effective control strategies, pharmaceutical scientists can maintain the purity, safety, and efficacy of Buspirone. Beyond regulatory obligations, impurity profiling advances scientific knowledge and reinforces the reliability of pharmaceutical products throughout their lifecycle.
