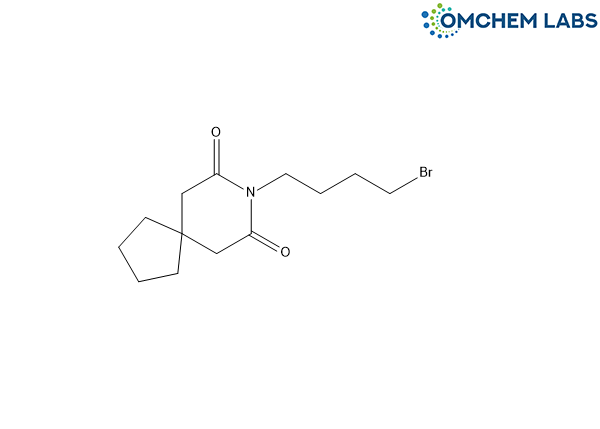
Buspirone Impurity M
| Catalogue No |
BUSP-OCL-003 |
| CAS NO |
80827-62-9 |
| Molecular Formula | C13H20BrNO2 |
| Molecular weight | 302.20 |
| Inquiry Status | In Stock |
| Synonyms | 8-(4-Bromobutyl)-8-azaspiro[4.5]decane-7,9-dione |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Buspirone Impurity M: A Scientific Overview
Introduction
The study of Buspirone Impurity M plays a critical role in modern pharmaceutical quality assurance. Impurities, even in trace amounts, can influence the overall safety, therapeutic effectiveness, and stability of drug substances. For active pharmaceutical ingredients (APIs) such as Buspirone, the presence of impurities must be thoroughly investigated and controlled in alignment with regulatory requirements. Profiling of Buspirone Impurity M contributes to understanding its origin, pathways of formation, and effective strategies for detection, isolation, and management. Such insights not only enhance drug quality but also strengthen regulatory compliance and patient safety.
Formation of Impurities During API Synthesis
Impurities like Buspirone Impurity M can arise from multiple stages in the chemical synthesis of the parent API. Potential contributors include incomplete reactions, residual intermediates, unintended by-products, or the use of catalysts and solvents. In some cases, storage conditions such as temperature fluctuations, moisture, or oxidative stress may also generate additional degradation products. The complexity of Buspirone’s synthetic pathway increases the likelihood of multiple impurity sources, making comprehensive monitoring essential. Recognizing these potential origins provides a foundation for effective impurity control strategies.
Analytical Data Interpretation Techniques
Accurate profiling of Buspirone Impurity M requires the application of advanced analytical technologies. Chromatographic methods such as high-performance liquid chromatography (HPLC) and gas chromatography (GC) are frequently employed to separate impurities, while hyphenated techniques like LC-MS and GC-MS provide structural insights. Spectroscopic methods, including NMR and IR, contribute to understanding functional group arrangements and structural identity. Interpretation of analytical data involves evaluating chromatographic retention, spectral signatures, and fragmentation patterns to differentiate Buspirone Impurity M from the main compound and other related substances. This multi-layered approach ensures both qualitative and quantitative assessment of impurity content.
Method Validation for Impurity Detection
The reliability of impurity profiling depends heavily on the validation of analytical methods. For Buspirone Impurity M, validation ensures that the applied techniques are accurate, reproducible, and capable of detecting impurities at trace levels. International guidelines, such as those outlined in ICH recommendations, define validation parameters including specificity, precision, accuracy, linearity, and sensitivity. Establishing these parameters builds confidence in the method’s suitability for routine impurity monitoring. A validated procedure thus provides regulatory assurance while supporting internal quality control systems.
Purification Strategies for Reducing Impurities
The removal or reduction of Buspirone Impurity M requires the use of tailored purification strategies. Crystallization remains a widely practiced method when solubility differences allow separation of impurities from the desired product. Chromatographic purification, including preparative HPLC, offers higher resolution for separating closely related compounds. Solvent extraction and distillation can be effective when impurities differ significantly in volatility or polarity. Selecting the appropriate purification approach depends on the chemical properties of Buspirone Impurity M and the desired yield of the final API. By employing efficient purification measures, manufacturers can achieve high levels of purity without compromising process efficiency.
Isolation and Characterization of Impurities
When Buspirone Impurity M exceeds regulatory thresholds or remains structurally ambiguous, it must be isolated and characterized. Isolation techniques such as preparative chromatography or fractionation enable the collection of sufficient quantities for detailed study. Structural characterization is then performed using techniques like NMR, MS, and IR spectroscopy, which reveal molecular architecture and functional groups. Characterization is vital not only for structural confirmation but also for assessing potential toxicological risks and determining qualification requirements. Developing reference standards for recurring impurities further enhances long-term quality assurance.
Conclusion
The profiling of Buspirone Impurity M exemplifies the importance of impurity management in pharmaceutical development. From its origins during synthesis to its final structural elucidation, each stage in impurity control reflects a commitment to safety, efficacy, and compliance. Through advanced analytical interpretation, validated detection methods, effective purification, and rigorous characterization, manufacturers can ensure that Buspirone meets the highest standards of quality. A comprehensive impurity profiling framework ultimately protects patient health while strengthening regulatory trust in the pharmaceutical product lifecycle.
