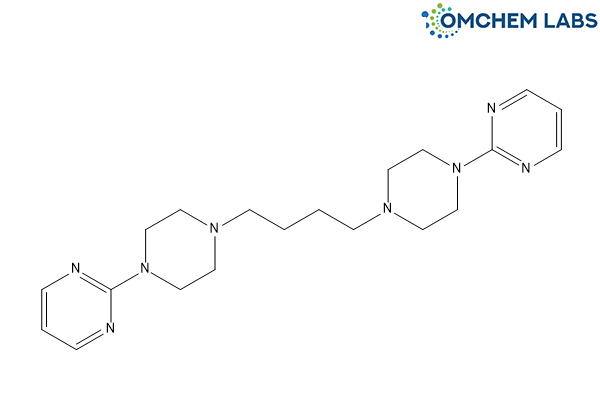
Buspirone Impurity C
| Catalogue No |
BUSP-OCL-005 |
| CAS NO |
257877-45-5 |
| Molecular Formula | C20H30N8 |
| Molecular weight | 382.51 |
| Inquiry Status | In Stock |
| Synonyms | Bispyrimidinylpiperazinyl Butane Impurity 2,2′-[Butane-1,4-diylbis(piperazine-1,4-diyl)]dipyrimidine |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Buspirone Impurity C: A Scientific Perspective
Introduction
The pharmaceutical industry places immense importance on understanding impurities associated with active pharmaceutical ingredients (APIs). Among these, Buspirone Impurity C serves as an example of how even minor chemical variations in a drug substance can carry significant implications for safety, stability, and efficacy. Impurity profiling involves the systematic study of such by-products to ensure that they remain within acceptable limits, in accordance with global regulatory frameworks. The development of robust impurity control strategies is not only a regulatory necessity but also an essential element in maintaining consistent therapeutic performance.
Formation of Impurities During API Synthesis
The generation of impurities in Buspirone Impurity C can occur through multiple pathways during synthesis and subsequent handling. These include incomplete reactions, transformation of intermediates, interactions of starting materials with reagents, or side reactions induced by reaction conditions such as temperature or pH. Additionally, degradation during storage, influenced by moisture, oxygen, or light, can contribute to the impurity profile. Each stage of synthesis, from raw material preparation to final crystallization, offers potential points where chemical species may deviate from the intended reaction pathway, giving rise to structurally related impurities.
Analytical Data Interpretation Techniques
Profiling impurities in Buspirone Impurity C demands the use of advanced analytical platforms. Chromatographic methods like high-performance liquid chromatography (HPLC) and gas chromatography (GC) are frequently combined with spectroscopic techniques such as nuclear magnetic resonance (NMR), infrared (IR) spectroscopy, and mass spectrometry (MS). These tools enable researchers to detect, identify, and classify impurities with high precision. Interpretation of the resulting chromatograms, spectra, and fragmentation patterns allows scientists to build a comprehensive picture of the impurity landscape, ensuring that unidentified or unexpected signals are investigated and understood before product release.
Method Validation for Impurity Detection
The reliability of impurity analysis relies heavily on thorough validation of the analytical methods employed. For Buspirone Impurity C, validation confirms that chosen methods are accurate, precise, and reproducible, while remaining sensitive enough to detect impurities at very low concentrations. Parameters such as specificity, linearity, limit of detection, and robustness are carefully assessed to confirm the suitability of the technique. By meeting international guidelines for validation, the integrity of analytical data is ensured, strengthening both regulatory submissions and internal quality assurance frameworks.
Purification Strategies for Reducing Impurities
Effective purification plays a crucial role in controlling impurity levels in Buspirone Impurity C. Different strategies are employed depending on the chemical properties of the impurity and the API. Crystallization may exploit solubility differences to separate impurities, while distillation assists in the removal of volatile contaminants. Solvent extraction and preparative chromatography offer additional options for more complex impurity profiles. An optimized purification approach balances efficiency with product yield, while ensuring that the desired compound is isolated at the highest possible level of purity.
Isolation and Characterization of Impurities
When impurities surpass reporting or qualification thresholds, they must be isolated and characterized in detail. For Buspirone Impurity C, this often involves preparative-scale separation methods, followed by structural elucidation using NMR, MS, or IR techniques. The characterization process provides insight into the molecular structure of impurities, allowing toxicological assessments and supporting regulatory documentation. Establishing reference standards for recurring impurities further aids in routine monitoring and ensures reproducibility across multiple production batches.
Conclusion
The study of Buspirone Impurity C illustrates the multifaceted nature of impurity profiling in pharmaceutical research. From synthesis and formation pathways, through analytical interpretation and validation, to purification and isolation, each step contributes to a comprehensive impurity management strategy. Such work ensures compliance with international regulatory expectations while safeguarding patient health and maintaining therapeutic reliability. Developing a deep understanding of impurities not only strengthens product quality but also enhances confidence in the long-term performance of the pharmaceutical compound.
