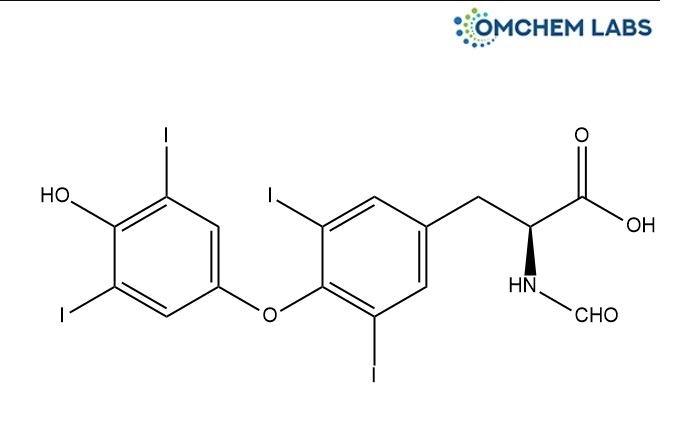
Levothyroxine N-Formyl Impurity
| Catalogue No |
LEVO-OCL-010 |
| CAS NO |
671235-41-9 |
| Molecular Formula | C16H11I4NO5 |
| Molecular weight | 804.88 |
| Inquiry Status | In Stock |
| Synonyms | N-Formyl-O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-L-tyrosine |
Detailed Overview of this Impurity: Discover more about Impurity Standard & Analysis
Impurity Profiling of Levothyroxine N-Formyl Impurity
Introduction
In the realm of pharmaceutical synthesis, the presence and understanding of impurities are essential for ensuring the therapeutic reliability of active pharmaceutical ingredients (APIs). Levothyroxine N-Formyl Impurity, as a potential by-product associated with the manufacture of levothyroxine, requires close scrutiny to meet stringent regulatory expectations and ensure patient safety. Impurities, whether inherent to the synthetic pathway or arising from environmental and procedural variables, must be profiled with scientific rigor. A well-established impurity profile supports product consistency, reinforces quality assurance, and contributes to the overall safety assessment of the pharmaceutical substance.
Formation of Impurities During API Synthesis
The development of Levothyroxine N-Formyl Impurity is typically associated with multiple variables during the synthetic process. Impurities can originate from incomplete reactions, intermediate instability, over-processing, or interactions with solvents and reagents. Reagent excess, reaction duration, and temperature fluctuations may foster the conversion of parent or intermediate molecules into unintended structures. Additionally, exposure to external factors such as light, air, and moisture during storage or handling can prompt degradation reactions that introduce structurally modified impurities. Each of these pathways underscores the importance of controlled synthesis and storage conditions to minimize impurity formation.
Analytical Data Interpretation Techniques
Comprehensive analysis of Levothyroxine N-Formyl Impurity involves the application of a suite of analytical tools designed to detect and differentiate minor components within a complex mixture. Techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and liquid chromatography–mass spectrometry (LC-MS) are instrumental in separating and identifying impurity signals. Spectroscopic methods, including nuclear magnetic resonance (NMR) and infrared spectroscopy (IR), support structural elucidation through molecular fingerprinting. Interpreting data from these platforms requires an integrated understanding of retention patterns, spectral peaks, and fragmentation behavior to accurately profile the impurity and its origin.
Method Validation for Impurity Detection
Establishing a validated methodology for impurity detection is essential to guarantee analytical reliability. Validation efforts focus on confirming that the selected techniques are suitable for detecting Levothyroxine N-Formyl Impurity with consistency and specificity. Core validation parameters include the method's ability to distinguish the impurity from the active ingredient, its reproducibility under varied conditions, and its performance across different sample matrices. This process ensures that the analytical approach remains robust, yielding dependable results across development, scale-up, and commercial production phases.
Purification Strategies for Reducing Impurities
Minimizing or eliminating Levothyroxine N-Formyl Impurity from the final API product involves carefully tailored purification strategies. The choice of purification technique is influenced by the impurity’s physicochemical characteristics and its relationship to the primary compound. Processes such as recrystallization, solvent exchange, phase separation, and preparative chromatography are commonly employed. These methods aim to exploit solubility differences, polarity, or molecular size to selectively remove impurities. A strategically optimized purification workflow not only improves API purity but also enhances yield and overall manufacturing efficiency.
Isolation and Characterization of Impurities
In scenarios where Levothyroxine N-Formyl Impurity is present above a defined threshold or remains unidentified, isolation and full characterization become critical. Isolation is typically achieved using scaled-up chromatographic methods designed to separate the impurity in sufficient quantity for further study. Characterization techniques, including mass spectrometry, NMR, and IR, are then applied to uncover the impurity’s structural and functional identity. These findings inform risk assessments, toxicological evaluations, and regulatory documentation, allowing for a well-documented impurity profile that aligns with international quality standards.
Conclusion
The comprehensive profiling of Levothyroxine N-Formyl Impurity is a cornerstone of quality assurance in pharmaceutical development. By integrating synthetic control, sophisticated analytical interpretation, validated detection methods, efficient purification, and precise structural identification, manufacturers can ensure consistent quality and regulatory compliance. This impurity profiling framework not only safeguards product performance but also reinforces the scientific integrity of pharmaceutical manufacturing practices. Ongoing refinement of these methodologies continues to elevate the standards for impurity control across the industry.
